|
 |
|
Esta página no está disponible en español. THE ORLANDO SENTINEL New Drug Trial Could Give MS Victims Hope By Stephanie Erickson April 17, 2003
Maria Rios' most agonizing moments don't come when the multiple sclerosis shoots fiery pain into her limbs or when it hurts her to swallow. It's not when she can fold just three shirts from the laundry before she must climb into bed, exhausted. The toughest times come when the lesions on her brain attack not her muscles, but her mind -- when she has to step out of line at the bank because she can't remember how to fill out a deposit slip, or when she's driving and suddenly can't remember where she's going. "Please, please help me," the devout Roman Catholic prayed one day when she got lost less than a mile from her east Orange County home. But a new drug is giving Rios, who moved to Orlando four years ago from Puerto Rico, new hope that the neurological disease won't permanently blind or paralyze her or destroy her mind. The 38-year-old mother will have the drug flowing through her veins next month. Orlando is one of a dozen cities currently screening people with MS for a nationwide study of a drug called Campath-1H. It could not only halt the symptoms of MS but could spare patients the inconvenience and pain of regular injections. The drug is approved by the U.S. Food and Drug Administration to treat some types of leukemia, but scientists have shown it may work wonders on people who have MS, a disease that has no cure and is the most common cause of disability in younger people. MS progressively destroys myelin, the covering that protects nerve fibers in the brain and spinal cord. The destroyed areas cause symptoms ranging from numbness, tingling and paralysis to fatigue, loss of vision and slurred speech. Most people who have MS live productive lives while being treated for symptoms. Patients who have the mild form experience flare-ups that last for days or months, followed by a nearly complete recovery. Over time, many patients evolve into a more progressive form, slowly losing more and more function. MS drugs now on the market require patients to inject themselves with shots, sometimes all over their bodies. The drugs typically prevent relapses in only 30 percent of patients. A small study showed that Campath is effective in more than 95 percent of MS patients and could stop the progression of MS altogether. Gone are daily injections. Patients get an intravenous infusion of the drug for half a day, five days in a row. They don't need it again for another year. "It's extremely exciting," said Dr. Daniel Jacobs, an Orlando neurologist. Campath works by destroying certain cells in the body that researchers think are responsible for MS. MS, which begins most often in people between ages 20 and 40, is thought to be an autoimmune disorder in which the immune system mistakenly targets portions of myelin as foreign and periodically tries to get rid of it. An estimated 400,000 Americans have the disease. Rios, one of 9,000 Central Floridians with MS, will be one of about 180 people nationwide participating in the new three-year study of Campath. The study will compare two doses of Campath to a drug called Rebif, considered the best FDA-approved drug available to reduce the number of MS flare-ups, Jacobs said. A successful study would result in one more, called a Phase III trial, to get FDA approval to treat MS patients. Patients randomly will get either Campath or Rebif. Rios, who sometimes forgets the names of her three daughters, smiles when she shares the news that she will be getting Campath. "Hopefully, by the third year I'll be feeling really, really good," she said. But Campath is not without side effects. The biggest risk is thyroid disease, so patients in the study will be screened along the way. Doctors say recruiting for the study has been challenging because the trial requires that patients have never before been treated for MS, even though they must have had at least two flare-ups. Some patients haven't been treated because they don't have insurance, while others don't want to take the shots, Jacobs said. Rios was just diagnosed in June but hadn't been treated because she went for a second opinion in November. That's when she met Jacobs, who told her about the study. She has had symptoms for years. When she was 9, she could barely walk. Doctors thought she had severe arthritis. Through the years, she coped with bad headaches and cramping in her limbs. Then came the dizziness, blurred vision and confusion. "It's like people would speak to me, but I wouldn't understand what they were saying," she said. On a magnetic-resonance image, a doctor discovered lesions on her brain, the telltale sign of MS. "It was devastating," she said. "One day you think you're fine, and the next day all this falls on you." Her life has changed quickly. She was an administrative assistant at Tru Green for four years, but she stopped working in January when she couldn't keep up with her duties. She walks with a cane, and she's in bed about 75 percent of the time now, watching reruns of The Golden Girls and Lifetime movies. Her husband, Freddie, and daughter, Crystal, 17, now have the chores of mopping the kitchen and cleaning the bathroom. Her 9-year-old daughter, Cristina, asks her, "Mommy, are you going to die?" Her third daughter is grown and lives elsewhere. Rios said she doesn't feel sorry for herself because she thinks God gave her the disease. "I've lived my life, my dreams came true," she said. "Only God knows if he put me on Earth to be in this study. If it works on me, it's wonderful, because it will work on other people."
|

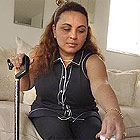 Waiting for relief. (GEORGE SKENE/ORLANDO SENTINEL)
Waiting for relief. (GEORGE SKENE/ORLANDO SENTINEL)